Bronchial Thermoplasty (BT):
Outpatient procedure proven to last for at least 5 years for patients with Severe Asthma1
Clinically effective
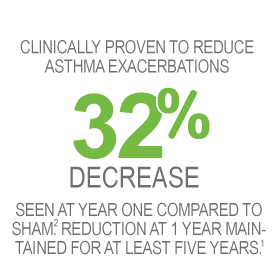
Proven to last at least 5 years
- Reduction in percentage of patients experiencing exacerbations seen at 1 year maintained for at least 5 years (primary endpoint).2
- Additionally, there was a 44% decrease (average over 5 years) in the percentage of BT-treated patients having severe exacerbations, compared with 12 months prior to BT treatment.2
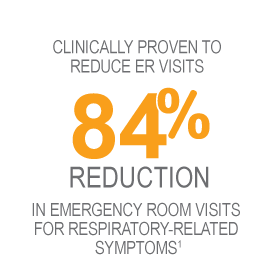
- Reduction in ER visits seen at 1 year maintained for at least 5 years.2
- Additionally, there was a 78% decrease (average over 5 years) in the percentage of BT-treated patients experiencing ER visits for respiratory symptoms, compared with 12 months prior to BT treatment.2
Asthma experts around the world talk about the effectiveness of Bronchial Thermoplasty
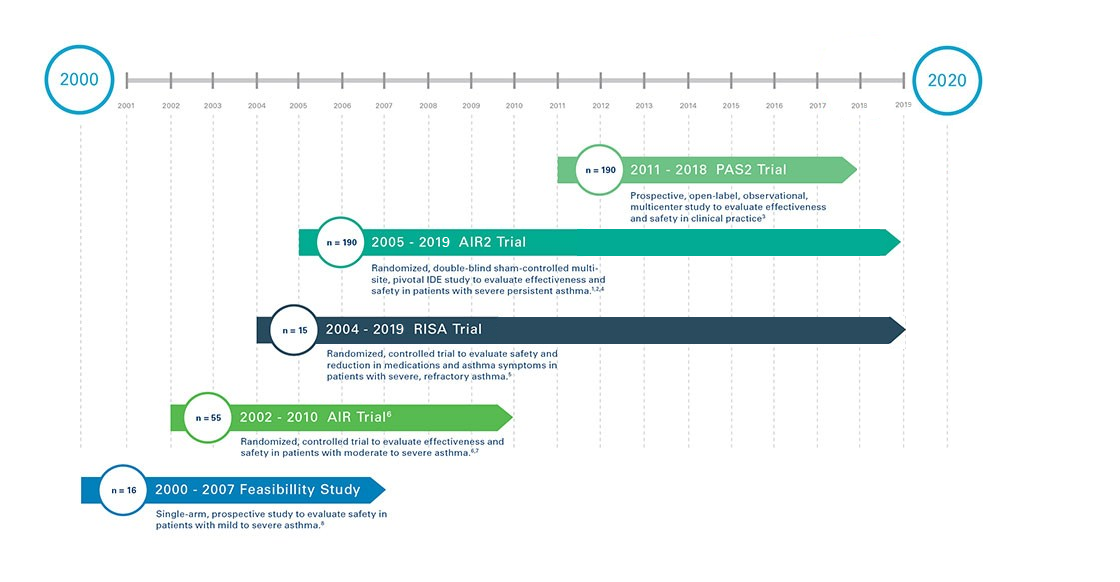
Robust Clinical Research
Bronchial Thermoplasty is supported by four completed clinical studies, and one ongoing, in asthma patients all with five year follow up: three of the trials were randomized, controlled, clinical trials.
Proven Safety
Asthma experts around the word say BT safe.
- No increase was seen in hospitalizations, asthma symptoms, or respiratory adverse events over the course of 5 years in patients treated with Bronchial Thermoplasty, delivered by the Alair System.2
- No structural changes in the airways that were clinically significant were due to BT at 5 years.2
- Absence of clinically significant deterioration in lung function (pre-bronchodilator FEV1 at 5 years.2
Understand the risks of the Bronchial Thermoplasty procedure
As with any procedure, there are risks, and individual results may vary. The most common side effect of BT is temporary worsening of respiratory-related symptoms. This side effect typically occurs within a day of the procedure and resolves within 7 days on average with standard care. There is a small (3.4% per procedure) risk of these symptoms requiring hospitalization.1

We’re all in.
For you, for your patients.
Click below to be contacted by a representative